Arineta Receives FDA Clearance for its Deep Learning Image Reconstruction Technology in SpotLight™ Cardiovascular CT Scanners
DLIR enhances image quality with reduced noise and low dose for cardiac, vascular and thoracic clinical procedures
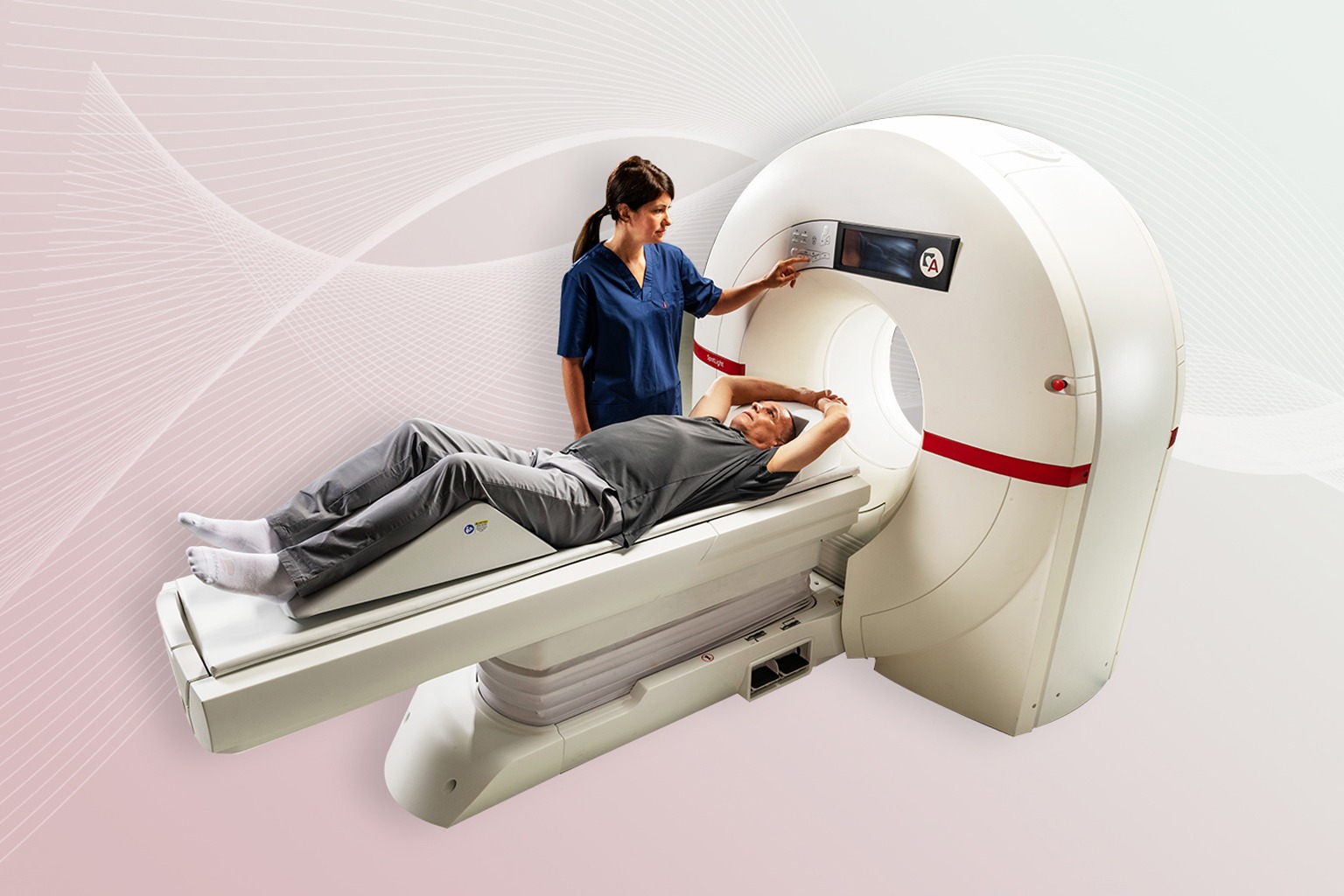
Caesarea, Israel – November XX, 2023 – Arineta Cardio Imaging, a leader in point-of-care cardiovascular CT solutions, today announced 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its deep learning image reconstruction (DLIR) technology for use in its SpotLight™ family of cardiovascular CT (CCT) scanners. This next-generation image reconstruction technology, powered by artificial intelligence, allows Arineta to provide enhanced image quality and image noise reduction to its customers.
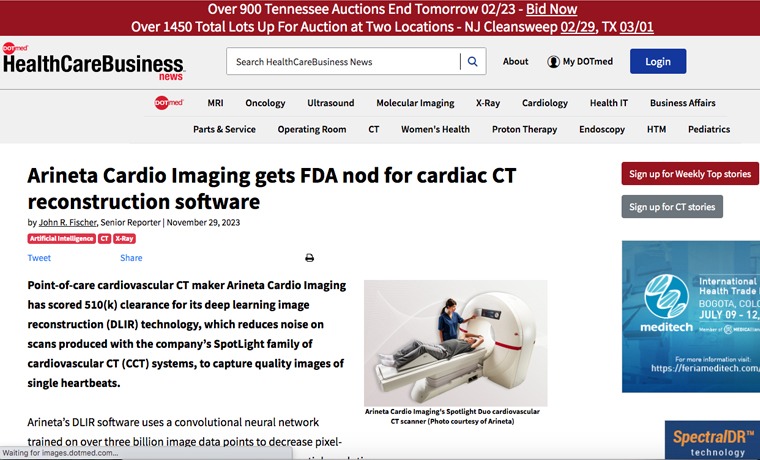
DLIR utilizes an advanced deep learning convolutional neural network (CNN) that was trained on more than 3 billion image data points. In comparison to prior technology, DLIR decreases pixel-wise noise magnitude without reducing high-contrast spatial resolution.
DLIR is available on both of Arineta’s 2nd generation systems, including the SpotLight dedicated cardiovascular CT and SpotLight Duo cardio-thoracic CT systems. SpotLight CT systems with DLIR are indicated for diagnosis of disease or abnormality and for planning of therapy procedures.
“We have used Arineta cardiac CT systems for several years, and they provide the highest quality cardiac CT clinical images for our practice,” said Matthew Budoff MD, Professor, Endowed Chair of Preventive Cardiology at Harbor-UCLA. “From our FDA 510(k) reader study, Arineta’s DLIR technology continues that excellence. Arineta’s SpotLight systems make the highest performance cardiac CT available at point-of-care, in an office, mobile, or cath lab setting.”
“Today, fewer than 5% of US patients that need cardiac CT are getting appropriately scanned due to lack of access at point-of-care”, said Arineta CEO Scott Schubert. “This FDA clearance continues Arineta’s vision and leadership to make cardiac CT the front-line imaging test for patients with chest pain and suspected cardiovascular disease, as recommended by the ACC & AHA 2021 guidelines.”
About Arineta
Arineta Cardio Imaging is an Israeli company that develops and produces innovative cardiovascular imaging solutions. Arineta is dedicated to growing cardiac CT as the front-line non-invasive test for diagnosing, therapy planning and monitoring of cardiovascular disease, the no. 1 cause of death and costs for healthcare worldwide. Arineta’s flagship product, SpotLight™ Duo, is the world’s first dedicated single-heartbeat, whole-heart cardiovascular and thoracic CT, based on the company’s proprietary Stereo CT technology. Among Arineta’s scientists are the inventors of the multi-slice spiral CT technology, still the cornerstone of CT imaging.
For more information, visit arineta.com.
Read all the related Press clippings here:
© 2023 Arineta Ltd, All Rights Reserved